Abstract
Introduction
Treatment with the BCL-2 inhibitor Venetoclax (VEN) in combination with hypomethylating agents (HMA) or low-dose cytarabine (LDAC) has shown encouraging results in patients with acute myeloid leukemia (AML). In contrast to the FDA, EMA approval for unfit AML patients was given only recently, and VEN combinations have therefore often been used as off-label treatment for relapsed/refractory (r/r) AML patients. We conducted a retrospective study of 73 unfit or r/r AML patients treated with a VEN combination between 2017 and 2021 at two German university hospitals.
Methods
Data was collected by medical chart review and included genetics, ELN2017 risk classification, previous treatment lines, courses of VEN treatment as well as outcome. All statistical tests were performed with GraphPad Prism. The median time of follow-up was 8.3 months.
Results
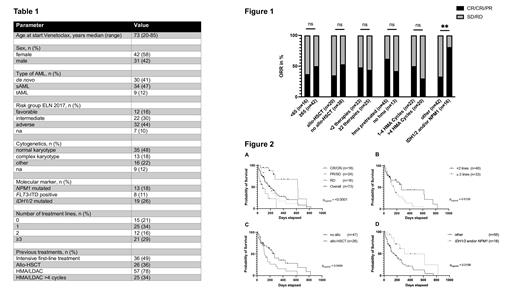
At beginning of VEN treatment, the median age was 73 (20-85) years (Table 1). The majority of patients had a secondary (s) AML [n=34 (47%)] and was assigned to the adverse ELN2017 risk group [n=32 (44%)]. Mutations in isocitrate-dehydrogenase 1/2 genes (IDH1/2) were the most frequent alteration [n=19 (26%)]. Before VEN treatment, a total of n=58 (79%) patients had received prior treatment including intensive chemotherapy [n=36 (49%)] and allogeneic stem cell transplantation (allo-HSCT) [n=26 (36%)]. Twenty-five (34%) patients were treated with >4 cycles HMA or LDAC before VEN initiation. VEN was given as first-line treatment in n=15 (21%) patients and started during the first or second treatment cycle of HMA/LDAC.
The initial VEN dosage after ramp-up during cycle 1 was in median 400mg (50-800mg). Patients received VEN in combination with azacytidine [n=34 (47%)], decitabine [n=18 (25%)] or LDAC [n=20 (28%)]. In median, patients had received 3 (1-17) VEN cycles at data cut-off. VEN was initiated after progression on HMA/LDAC treatment (>2 cycles) in n=35 (48%) patients. In most patients VEN was discontinued or dose-adjusted during treatment [cycle 1 n=37 (51%), after cycle 1 n=43 (59%)].
Response assessment was available for n=58 (79%) patients, of which n=18 (25%) achieved complete remission (CR) or CR with incomplete hematologic recovery (CRi), n=24 (33%) a partial remission (PR) or stable disease (SD), and n=16 (22%) were refractory to VEN combination treatment. The overall response rate (ORR) including CR/CRi/PR patients was 47% and not influenced by age or previous treatments including allo-HSCT and HMA pretreatment. Achievement of CR/CRi was significantly better in patients with IDH1/2 and/or NPM1 mutations (Figure 1).
The median overall survival (OS) of the entire cohort was 6.5 months. OS was significantly better in patients achieving a CR/CRi (20.3 months) as compared to patients with PR/SD/RD. (p<0.0001; Figure 2 A). OS was shorter in patients with more than two prior treatment lines (p=0.01, Figure 2 B) and in patients who had received allo-HSCT (p=0.05, Figure 2 C). There was no significant impact on OS with respect to age (>=65 years), ELN2017 risk group or previous HMA treatment. OS was however significantly longer in patients harboring NPM1 and/or IDH1/2 mutations (p=0.016; Figure 2 D).
Conclusions
Our real-world analysis demonstrates that VEN combination treatment is feasible and effective also in r/r AML patients. Response rates and survival were lower than in patients treated with VEN combinations in first line (DiNardo, NEJM 2020) and in our cohort highly influenced by the number of previous treatment lines. As in patients treated with VEN combinations at fist line, the NPM1 and IDH1/2 genotype was associated with better response and survival. Further studies with larger cohorts are needed to investigate the role of VEN combinations in r/r AML.
Westermann: Abbvie: Consultancy, Honoraria; Astellas: Honoraria; Novartis: Consultancy, Honoraria; BMS: Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Stem Cell Line: Consultancy, Honoraria. Bullinger: Seattle Genetics: Honoraria; Bayer: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Astellas: Honoraria; Amgen: Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Gilead: Consultancy; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Menarini: Consultancy; Sanofi: Honoraria; Celgene: Consultancy, Honoraria; Hexal: Consultancy. Keller: Abbvie: Other: Advisory Role. Krönke: BMS/Celgene: Other: Advisory board; Abbvie: Other: Advisory board. Goetze: Abbvie: Other: Advisory Board; BMS/Celgene: Other: Advisory Board, Research Funding.
Venetoclax was used "off-lable" in unfit and relapsed/refractory AML patients in combination with HMA/LDAC.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal